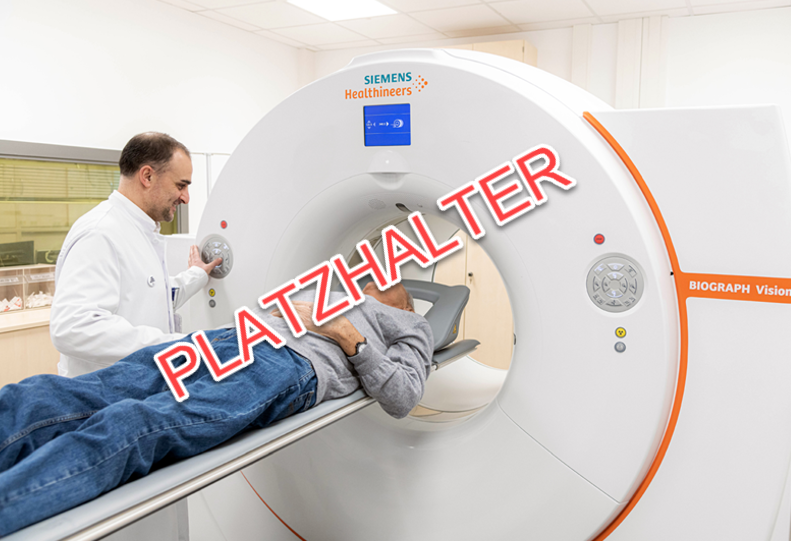
What we do
Research cooperation
•The radiopharmacy department has a variety of validated automated synthesis platforms that allow the development of GMP-compliant automated syntheses and analytics from “bench to manufacturing authorization”.
•Development of new synthesis methodologies that allows fast and efficient chemical incorporation of radionuclides in target molecules as well as optimal purification and formulation strategies.
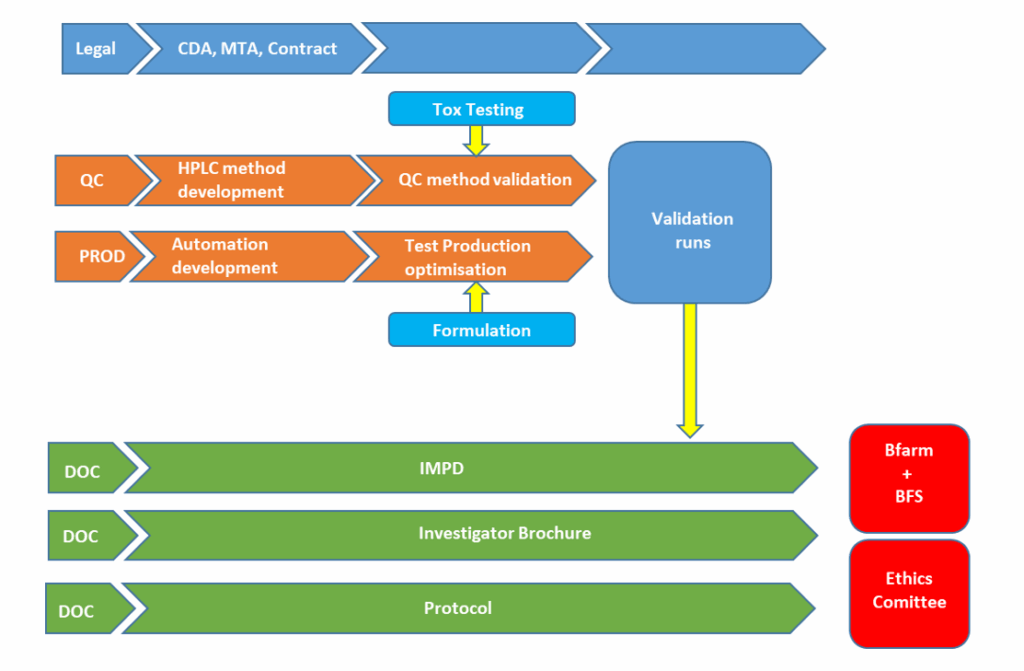
•After successful non-clinical validation and tox testing, the tracer can be advanced to the clinic and becomes a radiopharmaceutical.
•After the set up of the regulatory documentation such as IMPD, investigator Brochure and study protocol the clinical trial application is submitted to the Federal Institute for Drugs and Medical Devices (Bfarm).
Contract Manufacturing
- The radiopharmacy department is manufacturing partner for
clinical projects using PET and therapy tracers. - We offer a one-stop-shop for early and late stage clinical
manufacturing - The Radiopharmacy Department has a professional CMC team
that ensures a smooth technology transfer in compliance with current GMP guidelines
Tracer Production
- Our production follows cGMP and is in accordance with the EU-GMP-Guideline Part II (ICH Q7). We are regularly inspected by the responsible local authority, which issues us with drug substance-specific GMP certificates.
- The department has a marketing authorization for the product Flucose (active ingredient: 18F-FDG).
- The current product list includes 30 radiopharmaceuticals for therapy and diagnostics that are produced under manufacturing authorization, within clinical trials or according to AMG §13(2b).

