
Präklinische Theranostik
(Preclinical Theranostics)
Research
Our research focuses on theranostics with the goal to deliver new insights into tumor biology, heterogeneity and metabolism, and their relationship and relevance to functional imaging and biomarker-driven treatments in nuclear medicine.
The term theranostics combines the words diagnosis and therapy; it represents a precision medicine approach relying on a specific targeted diagnostic test that helps to select patients for a specific targeted therapy. In nuclear medicine, radioligands are used for theranostics. Radioligands are radio-labelled molecules that bind to a cell surface receptor on target cells. When coupled to radio-isotopes like Gallium-68 or Fluor-18, radioligands can be used for imaging with positron emission tomography (PET), single-photon emission computed tomography (SPECT), or scintigraphy. This imaging facilitates the selection of patients for subsequent radioligand therapy. Radioligand therapy is an emerging treatment modality for various cancers. In radioligand therapy, the same or a very similar radioligand as that used for imaging is coupled to a therapeutic radioisotope (e.g., Actinium-225, Lutetium-177, Yttrium-90) to deliver ionizing radiation specifically to tumors. Radioligand therapy can thus be described as a type of targeted, systemic radiotherapy. In contrast to conventional radiotherapy, radioligand therapies are systemically administered, and radioligands are internalized upon binding to tumor cells. As a consequence, metastases throughout the body can be targeted, and tumors are irradiated for hours to days.
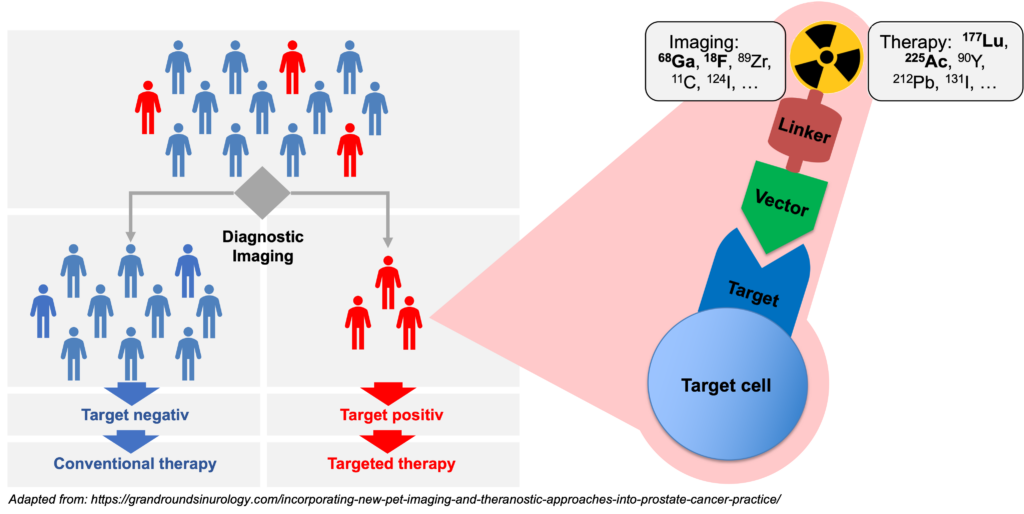
Molecular imaging in nuclear medicine combines imaging modalities like PET and SPECT with computed tomography (CT) or magnet resonance tomography to derive detailed information on disease. In the clinic, molecular imaging is mainly used for diagnosis, staging, monitoring response to therapy (e.g., by pre- and post-therapeutic FDG-PET/CT), and to select patients for RLT (e.g., PSMA-PET/CT). Our group aims at establishing molecular imaging as a phenotypic biomarker, i.e., to connect imaging information with the tumor biology, metabolism and intra- and inter-individual tumor heterogeneity. Ultimately, this will provide imaging strategies that can guide selection of individualized – and therefore more effective and less toxic – treatment regimens that improve quality of life and overall survival of patients.
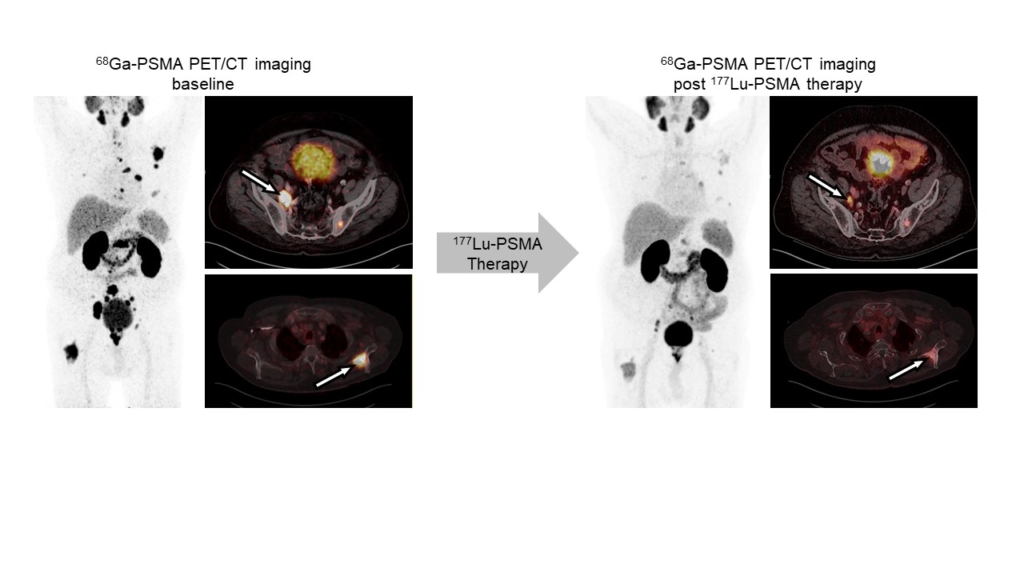
Radioligand therapy is a promising yet rarely curative oncological treatment option. Our research aims at identifying predictors (biomarkers) for and determinants (molecular and cellular processes) of response/non-response to radioligand therapy. We investigate tumor cell intrinsic resistance mechanisms, tumor protection by microenvironmental factors (immunosuppression, stroma), and suboptimal tumor radiation dose delivery using cancer models and patient samples, and diverse in vitro, ex vivo and in vivo analyses. We use the knowledge gained from these studies to rationally design and provide proof-of-principle for new radioligand therapy combination regimens that can be translated into the clinic.
Building on the first success stories of theranostics, we evaluate oncological targets and develop organic chemical or radiochemical processes for the production and development of new radioligands for imaging and therapy. This includes the design, chemical synthesis, radiolabeling, and in vitro, in vivo, and ex vivo evaluation of ligands.
The role of molecular imaging technologies in detecting, evaluating, and monitoring cardiovascular disease and their treatment is expanding rapidly. Gradually replacing the conventional anatomical or physiological approaches, molecular imaging strategies using biologically targeted markers provide unique insight into pathobiological processes at molecular and cellular levels and allow for cardiovascular disease evaluation and individualized therapy. We use SPECT and PET imaging to assess myocardial perfusion to find areas of damaged heart muscle and visualize the size and location of myocardial infarction. Specific radiotracers are used to assess cardiac inflammation and fibroblast activity in different cardiac disease models.

Prof. Dr.
Katharina Lückerath
Leiterin Präklinische Theranostik
Collaborations
Imaging / Theranostics resources for collaborators
- In vivo small animal imaging: We use the Molecubes β-Cube (PET), g-Cube (SPECT) and X-Cube (CT), specifically designed for rodent imaging. The scanners offer high resolution (spatial resolution: CT, 0.05 mm, PET, 0.85 mm, SPECT, 0.5 mm) and throughput imaging. PMOD (PFUS, PBAS, PKIN, PCARD) and Vivoquant are available for data Analysis
- Ex vivo biodistribution studies (Wizard 2480)
- Autoradiography (BetaIMAGER)
Interested in imaging or theranostics?
We’re happy to discuss collaborative projects.
Team
Präklinische Theranostik

Prof. Dr.
Katharina Lückerath
Leiterin Präklinische Theranostik

Lara Breuer
PhD student

Dr. rer. nat.
Yalcin Kuzay
Postdoc

Jasmin Wosniack
BTA

Dr. rer. nat.
Valeska von Kiedrowski
Senior scientist

Dr. rer. nat.
Justin Dubbert
Postdoc

Natalie Heiming
BTA

Anna Sickau
BTA
